Soon, Your Prescription Pills Could Include Tracking Sensors
For ALS Patients, Devices on the Horizon Make Communicating Easier
 The late theoretical physicist Dr. Stephen Hawking is widely regarded as one of human history’s great geniuses. Thankfully for us, he managed to communicate his genius despite suffering from amyotrophic lateral sclerosis, or ALS. ALS is a neurodegenerative brain condition that gradually weakens the patient’s motor neurons and makes it increasingly difficult to speak. Hawking typically spoke wi... Read More
The late theoretical physicist Dr. Stephen Hawking is widely regarded as one of human history’s great geniuses. Thankfully for us, he managed to communicate his genius despite suffering from amyotrophic lateral sclerosis, or ALS. ALS is a neurodegenerative brain condition that gradually weakens the patient’s motor neurons and makes it increasingly difficult to speak. Hawking typically spoke wi... Read MoreThis Minnesota Grandmother Invented a Medical Device with Stuff Around the House
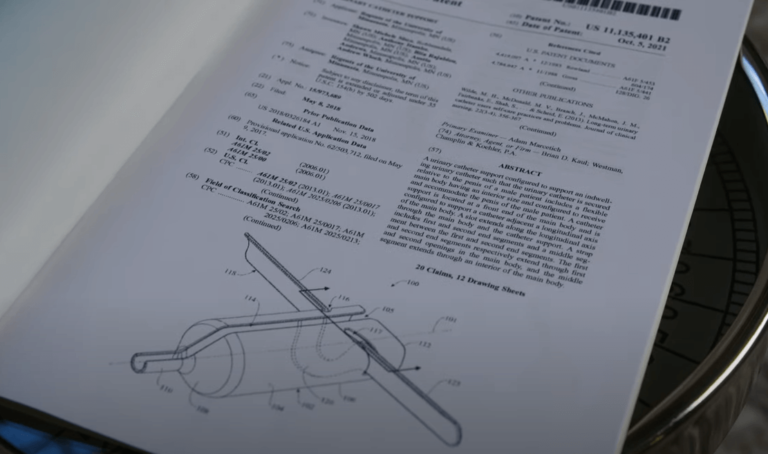 If you think you need to be an engineering or medical professional to invent a medical device, this story will change your mind. A Minnesota grandmother and part-time customer service worker named Shawn Sisco invented a device that prevents a common problem caused by Foley catheters. The tube of the Foley catheter has been known to bend or twist, abrading the skin of its user. It happened t... Read More
If you think you need to be an engineering or medical professional to invent a medical device, this story will change your mind. A Minnesota grandmother and part-time customer service worker named Shawn Sisco invented a device that prevents a common problem caused by Foley catheters. The tube of the Foley catheter has been known to bend or twist, abrading the skin of its user. It happened t... Read MoreWireless Charging in Medical Devices Becoming a Valued Feature
 For inventors of medical devices, it can be deeply gratifying when an invention improves the patient experience, bringing relief that can be psychological as well as physical. In a recent article published on Machine Design, Rehana Begg pointed out how electrical cords and wires on medical devices can produce anxiety for patients who are already in a state of discomfort and fear. Wired medical dev... Read More
For inventors of medical devices, it can be deeply gratifying when an invention improves the patient experience, bringing relief that can be psychological as well as physical. In a recent article published on Machine Design, Rehana Begg pointed out how electrical cords and wires on medical devices can produce anxiety for patients who are already in a state of discomfort and fear. Wired medical dev... Read MorePatented Cervical Artificial Disc Revolutionizes Spinal Injury Recovery
 A new medical device called Simplify® recently received approval from the U.S. Food and Drug Administration (FDA). This device is a cervical artificial disc meant to serve as an implant for people who whose existing cervical disc have become too narrow, causing pain in their spinal cord. This implant is placed into a patient’s spine through cervical disc arthroplasty, which originally began to ... Read More
A new medical device called Simplify® recently received approval from the U.S. Food and Drug Administration (FDA). This device is a cervical artificial disc meant to serve as an implant for people who whose existing cervical disc have become too narrow, causing pain in their spinal cord. This implant is placed into a patient’s spine through cervical disc arthroplasty, which originally began to ... Read More